- Visibility 84 Views
- Downloads 16 Downloads
- Permissions
- DOI 10.18231/j.ijashnb.2021.006
-
CrossMark
- Citation
Efficacy of platelet rich fibrin in osseous regeneration after periapical surgeries
- Author Details:
-
Parveen Akhter Lone *
-
Tasleem Kouser
-
Varun Salgotra
-
Majid Khan
Abstract
Aims & objectives: to evaluate the efficacy of platelet rich fibrin in soft tissue healing, bone regeneration & to assess its effect on post-operative pain & swelling after periapical surgeries
Materials and Methods: This prospective study was carried out in oral & maxillofacial surgery department. The study sample consists of 40 patients diagnosed with periapical pathologies of maxillary teeth only. Periapical curettage / enucleation & Apicoectomy was done in all patients & patients were divided into two groups. PRF placement was done in surgical cavity in Group A with 20 patients (Study group). In Group B with 20 patients, (control Group) surgical cavities were kept empty without PRF. Wound was sutured with 3.0 black silk. Pain, Swelling, soft tissue & bone healing was noted on 3rd & 7th day, 1, 3 6, 9 months post operatively
Results: Group a showed fast clinical & radiographic bone healing. Pain was less & consumption of analgesics was less as compared to Group B, however there was no difference in swelling & soft tissue healing in both group. Bone healing was complete in 6months in Group A
Conclusion: Use of PRF promoted good results in terms of clinical & radiographic healing
Introduction
Pulp necrosis followed by trauma or dental caries if left untreated may lead to periapical pathologies like peri apical granuloma & cystic lesions. Periapical surgery is indicated in cases where conventional root canal therapy fails.[1] The main cause of unsuccessful endodontic therapy is presence of infected tissue in endodontic spaces.[2] Periapical surgery is aimed to remove periapical pathology followed by complete soft tissue & radiographic bone healing. Successful endodontic therapy depends on complete periapical repair & regeneration of soft tissue & bone.[3], [4] The teeth undergoing apical surgery have less predictable prognosis, so the possibility of accelerating bone regeneration in surgical defects in periapical region is important, as even a single tooth can be strategic in prosthetic rehabilitation. Bone & soft tissue regeneration can be achieved by local application of hormones, growth factors & plasma derivatives as advocated by studies.[5] Bone morphogenic proteins, parathyroid hormone, platelet-derived growth factor, platelet rich plasma, & enamel matrix proteins have been applied to promote healing of surgical wounds /defects.[6], [7], [8], [9]
Platelet rich fibrin a biologic revolution constituted by fibrin network of platelets, leukocytes, cytokines, stem cells, & three dimensional architectures, which favours wound healing & immunity.[10] Platelets in PRF are capable of releasing platelet derived growth factor (PDGF) transforming growth factor β1 (TGF β1), insulin like growth factor (IGF) & exhibit varied potent local properties like cell migration, cell attachment, cell proliferation & cell differentiation even up to one week.[11], [12]
Materials and Methods
This prospective randomised, controlled clinical Study was performed in the department of OMFS. Study was conducted on 40 patients diagnosed with periapical lesions in maxilla. Patients were divided into two groups, Group A & B comprising of 20 patients each. Diagnosis was made on History, clinical & radiographic examination of patient.
Operative procedure
A Standard periapical surgery procedure was performed. After removal of periapical pathology by enucleation, curettage & Apicoectomy PRF clot was inserted in surgical cavity in Group A. Whereas in controlled group (Group B) surgical cavities were left empty. Flap closure was done using 3.0 black silk. Sutures were removed on seventh post-operative day. Post-operative analgesics, antibiotics & instructions were given to all patients
Preparation of PRF gel
PRF gel was prepared by protocol given by Choukroun et al [13]
1.5 ml of venous blood was drawn in a tube without anticoagulant & was immediately centrifuged at 3000rpm for 10 minutes .Blood clot was extracted using sterile forceps & separated into 3 layers.
Upper straw-coloured layer acellular plasma was removed.
The middle layer containing PRF was collected 2mm below the lower diving line.
Third red coloured layer, lower fraction containing red blood cells.
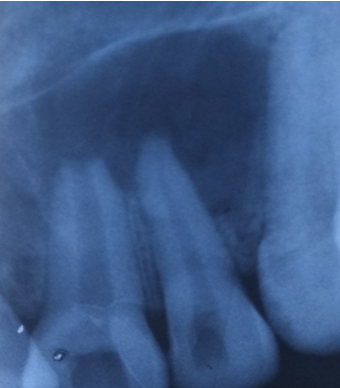

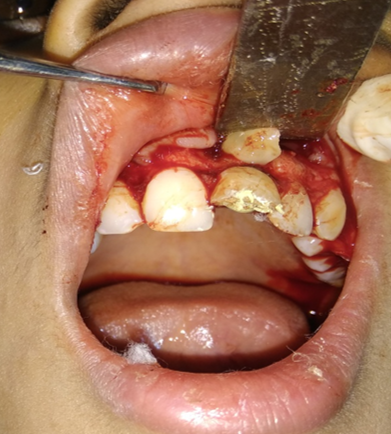
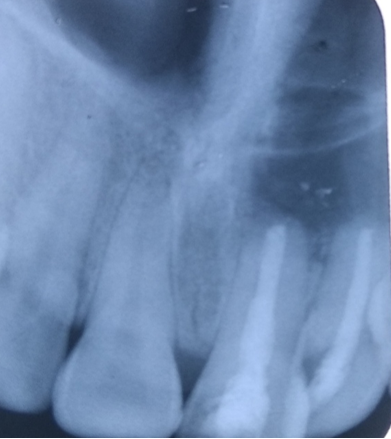
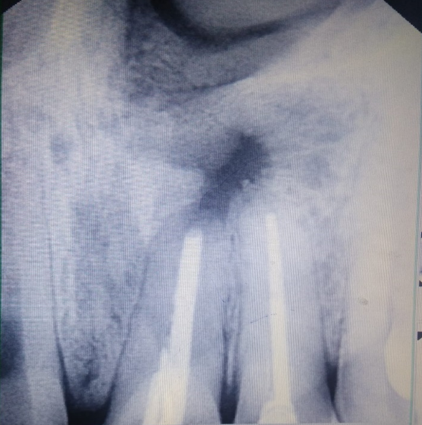
Follow up of the patient was done on 3rd, 5th & 7th day post operatively. Radiographic evaluation of bone regeneration was done in three, Six, nine & 12 months. No of analgesics consumed from first day to 7th day, swelling, healing of wound, any other complication was recorded during follow-up. Patients were given questionnaire & instructed to record their pain level using Visual Analogue Scale (VAS). Soft tissue healing was assessed by healing index reported by Landry et al.[14] which depends on tissue colour, presence of bleeding on palpation, epithelialization of wound margin granulation tissue & suppuration.
Results
In this study in PRF group pain was reported significantly less & patients consumed less analgesics compared to control group from third post-operative day on wards. Post-operative intra & Extra oral swelling was evaluated after 24, 48, 72 hours & 7th post op day. No difference was seen in swelling & soft tissue healing in both groups. At 3 months, PRF group exhibited better bone healing than control group. Bone density was assessed by comparing bone formation in both groups (PRF & Non PRF) using intra oral periapical radiographs & Orthopantomographs at first, 3rd third 6th , 9th & 12th months post operatively. Highly Significant bone formation was noted in PRF group at 3rd month & bone healing was almost complete on 6th month. Non-PRF group (Control) showed slow radiographic bone healing. Bone healing was complete between 9-12 months.
Discussion
Choukroun et al[13] in France first discovered platelet rich fibrin (PRF) an autologous fibrin matrix to enhance bone regeneration. PRF is easy, simple inexpensive process & requires no additive in comparison to platelet rich plasma (PRP). PRP requires artificial biochemical adding for modification & neutralising the anticoagulant. The addition of bovine derived thrombin to promote conversion of fibrinogen into fibrin is also eliminated.[15] PRF is a by -product of patients own blood therefore chances of transmission of infectious disease is rare & patients need not to bear the expense of harvesting procedure in hospital.[16]
The result of present study shows that PRF is efficient in decreasing pain & less consumption of analgesics by study group as compared to controlled group. This study is in accordance with the studies carried out by Kumar et al[17] & Ahmed et al[18] who also revealed that PRF significantly reduced post-operative pain & less analgesic consumption after third molar surgery.
In the present study intra, oral swelling was evaluated & classified as Mild, Moderate & sever according to clinical visual evaluation. Extra oral swelling was evaluated by tape measurement in horizontal direction from midline to last point of swelling. Measurement in vertical direction was done from supraorbital margins to maxillary occlusal plane. This study didn’t show any significant difference in post operatively intra & extra oral swelling between controlled & study group similar to the studies reported by Uyanik et al. [19]
Jeyaraj et al[20] & Marenzi et al[21] demonstrated better soft tissue healing clinically on 3,7, &14 days after surgery in study group in extraction sockets than controlled group. Contrary to this, present study did not show any difference in soft tissue healing in both groups.
The studies by Hauser & Gaydaro[22] demonstrated that there is increase in bone density from third to sixth month. Their analysis showed significantly improved architecture & higher bone quality in PRF Group .Similarly radiographic studies done by Alzahrani et al[23] showed significant difference in bone formation in study group at one, four & eight weeks. Choukroun & Diss indicated that when PRF membrane is used new blood vessels are generated & epithelialization is promoted which facilitates rapid wound coverage. The present study also showed marked bone healing of surgical cavity from 3rd month & at 6th month radiographic bone healing was complete in PRF group. In Non PRF group radiographic bone, healing of surgical cavities was complete between 9-12 months.
Simonpieri et al[24] reported that the use of platelet & immune concentrate offers following advantages 1) Fibrin clot plays important role & serves as biologic connectors between bone particles. 2) integration of this fibrin network into regenerative site facilitates cellular migration, particularly for endothelial cells necessary for neoangiogenesis & vascularization. 3) the platelet cytokines (PDGF, TGF-a, IGF-1) are released gradually as the fibrin matrix is resorbed thus creating a perpetual process of healing. 4) The presence of leukocytes & cytokines in the fibrin network also plays a significant role in self-regulation of inflammatory phenomenon within grafted material
Conclusion
The present study concluded that PRF is a part of new biotechnology & a healing biomaterial as it contains all the factors required for better & optimal consolidation of bone formation. The application of PRF gel made of intact platelets & fibrin by high-speed centrifuge provides an ideal scaffold for bony tissue repair in periapical surgeries. PRF placement showed promising result in stimulating bone formation after 3 months of surgery & reduced post-operative discomfort. However, Long-term follow up may be required to evaluate the final treatment outcome.
Acknowledgement
The authors thank & acknowledge the efforts of Ayesha Nisar, Ayera Bashir, Zain ul A Arifeen, & Maryam for their support in formulating the results
Source of Funding
No financial support was received for the work within this manuscript.
Conflict of Interest
The authors declare they have no conflict of interest.
References
- Mazumbar P, Bhunia S. Treatment of periapical lesion with platelet rich fibrin. Indian Med Gazette. 2013. [Google Scholar]
- Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15(6):348-81. [Google Scholar] [Crossref]
- Singh S, Singh A, Singh S, Singh R. Application of PRF in surgical management of periapical lesions. Natle J Maxillofac Surg. 2013;4(1):94-9. [Google Scholar]
- Mazumdar S, Joshi S, Ansari S. Experiences with the use of PRF (Plasma Rich Fibrin) in enucleated cystic cavity. J Indian Dent Assoc. 2014;8:19-26. [Google Scholar]
- Gassling V, Douglas T, Warnke P, Açil Y, Wiltfang J, Becker S. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res . 2010;21(5):543-9. [Google Scholar] [Crossref]
- Nevins M, Giannobile W, McGuire M, Kao R, Mellonig J, Hinrichs J. Platelet-Derived Growth Factor Stimulates Bone Fill and Rate of Attachment Level Gain: Results of a Large Multicenter Randomized Controlled Trial. J Periodontol. 2005;76(12):2205-15. [Google Scholar] [Crossref]
- Heijl L, Heden G, Svardstrom G, Ostgren A. Enamel matrix derivative (EMDOGAINR) in the treatment of intrabony periodontal defects. J Clin Periodontol . 1997;24(9):705-14. [Google Scholar] [Crossref]
- Jung R, Glauser R, Schärer P, Hämmerle C, Sailer H, Weber F. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implants Res . 2003;14(5):556-68. [Google Scholar] [Crossref]
- Johns D, Vidyanath S, Sam G, Shivashankar V. Combination of platelet rich fibrin, hydroxyapatite and PRF membrane in the management of large inflammatory periapical lesion. J Conserv Dent. 2013;16(3):261-4. [Google Scholar] [Crossref]
- Deenadayalan E, Kumar A, Tewari RK, Mishra SK, Iftekhar H. Management of large periapical lesion with the combination of second generation platelet extract and hydroxyapatite bone graft. A report of three cases. J Clin Diag. 2015;9:24-7. [Google Scholar]
- Johns D, Vidyanath S, Kumar M, Shivashankar V. Platelet Rich Fibrin in the revitalization of tooth with necrotic pulp and open apex. J Conserv Dent . 2012;15(4):395-8. [Google Scholar] [Crossref]
- Giannobile W, Somerman M. Growth and Amelogenin-Like Factors in Periodontal Wound Healing. A Systematic Review. Ann Periodontol . 2003;8(1):193-204. [Google Scholar] [Crossref]
- Choukroun J, Adda F, Schoeffel C, Vervelle A. Una opportunite` en paro-implantologie: le PRF. Implantodontie. 2001;42:55-62. [Google Scholar]
- Turnbull R, Howley T. Effectiveness of benzydamine HCL in treatment of periodontal post surgical patients. Res Cin Forum. 1988;10:105-18. [Google Scholar]
- Man D, Plosker H, Winland-Brown J. The Use of Autologous Platelet-Rich Plasma (Platelet Gel) and Autologous Platelet-Poor Plasma (Fibrin Glue) in Cosmetic Surgery. Plast Reconstr Surg. 2001;107:238-9. [Google Scholar] [Crossref]
- Jayadev M, Marshal V, Naik B, Karunakar P. Role of Platelet rich fibrin in wound healing: A critical review. J Conserv Dent. 2013;16(4):284-93. [Google Scholar] [Crossref]
- Kumar A, Tewari RK, Mishra SK, Iftekhar H. Management of large periapical lesion with the combination of second-generation platelet extract and hydroxyapatite bone graft: a report of three cases. J Clin Diagn Res. 2015;9(1):24-7. [Google Scholar]
- FA. Clinical effects of platelet-rich fibrin (PRF) following surgical extraction of the lower third molar. Saudi J Dent Res. 2017;8(1-2):19-25. [Google Scholar]
- Uyanık L, Bilginaylar K, Etikan �. Effects of platelet-rich fibrin and piezosurgery on impacted mandibular third molar surgery outcomes. Head Face Med. 2015;11(1):25-32. [Google Scholar] [Crossref]
- Jeyaraj P, Chakranarayan A. Soft tissue healing and bony regeneration of impacted mandibular third molar extraction sockets, following postoperative incorporation of platelet-rich fibrin. Ann Maxillofac Surg. 2018;8(1):10-8. [Google Scholar] [Crossref]
- Marenzi G, Riccitiello F, Tia M, Lauro A, Sammartino G. Influence of Leukocyte- and Platelet-Rich Fibrin (L-PRF) in the Healing of Simple Postextraction Sockets: A Split-Mouth Study. Bio Med Res Int. 2015;2015:1-6. [Google Scholar] [Crossref]
- Hauser F, Gaydarov N, Badoud I, Vazquez L, BJ, Ammann P. Clinical and Histological Evaluation of Postextraction Platelet-rich Fibrin Socket Filling. Implant Dent. 2013;22(3):295-303. [Google Scholar] [Crossref]
- Alzahrani A, Murriky A, Shafik S. Influence of platelet rich fibrin on post-extraction socket healing: A clinical and radiographic study. Saudi Dent J. 2017;29(4):149-55. [Google Scholar] [Crossref]
- Simonpieri A, Corso MD, Sammartino G, Ehrenfest D. The Relevance of Choukroun's Platelet-Rich Fibrin and Metronidazole During Complex Maxillary Rehabilitations Using Bone Allograft. Part II: Implant Surgery, Prosthodontics, and Survival. Implant Dent. 2009;18(3):220-9. [Google Scholar] [Crossref]
How to Cite This Article
Vancouver
Lone PA, Kouser T, Salgotra V, Khan M. Efficacy of platelet rich fibrin in osseous regeneration after periapical surgeries [Internet]. IP Indian J Anat Surg Head Neck Brain. 2021 [cited 2025 Oct 02];7(1):30-34. Available from: https://doi.org/10.18231/j.ijashnb.2021.006
APA
Lone, P. A., Kouser, T., Salgotra, V., Khan, M. (2021). Efficacy of platelet rich fibrin in osseous regeneration after periapical surgeries. IP Indian J Anat Surg Head Neck Brain, 7(1), 30-34. https://doi.org/10.18231/j.ijashnb.2021.006
MLA
Lone, Parveen Akhter, Kouser, Tasleem, Salgotra, Varun, Khan, Majid. "Efficacy of platelet rich fibrin in osseous regeneration after periapical surgeries." IP Indian J Anat Surg Head Neck Brain, vol. 7, no. 1, 2021, pp. 30-34. https://doi.org/10.18231/j.ijashnb.2021.006
Chicago
Lone, P. A., Kouser, T., Salgotra, V., Khan, M.. "Efficacy of platelet rich fibrin in osseous regeneration after periapical surgeries." IP Indian J Anat Surg Head Neck Brain 7, no. 1 (2021): 30-34. https://doi.org/10.18231/j.ijashnb.2021.006
